Vaccine Validity in Those Aged 5-11
Written By Mila Nguyen, Revised by Melanie Tran, November 15, 2021
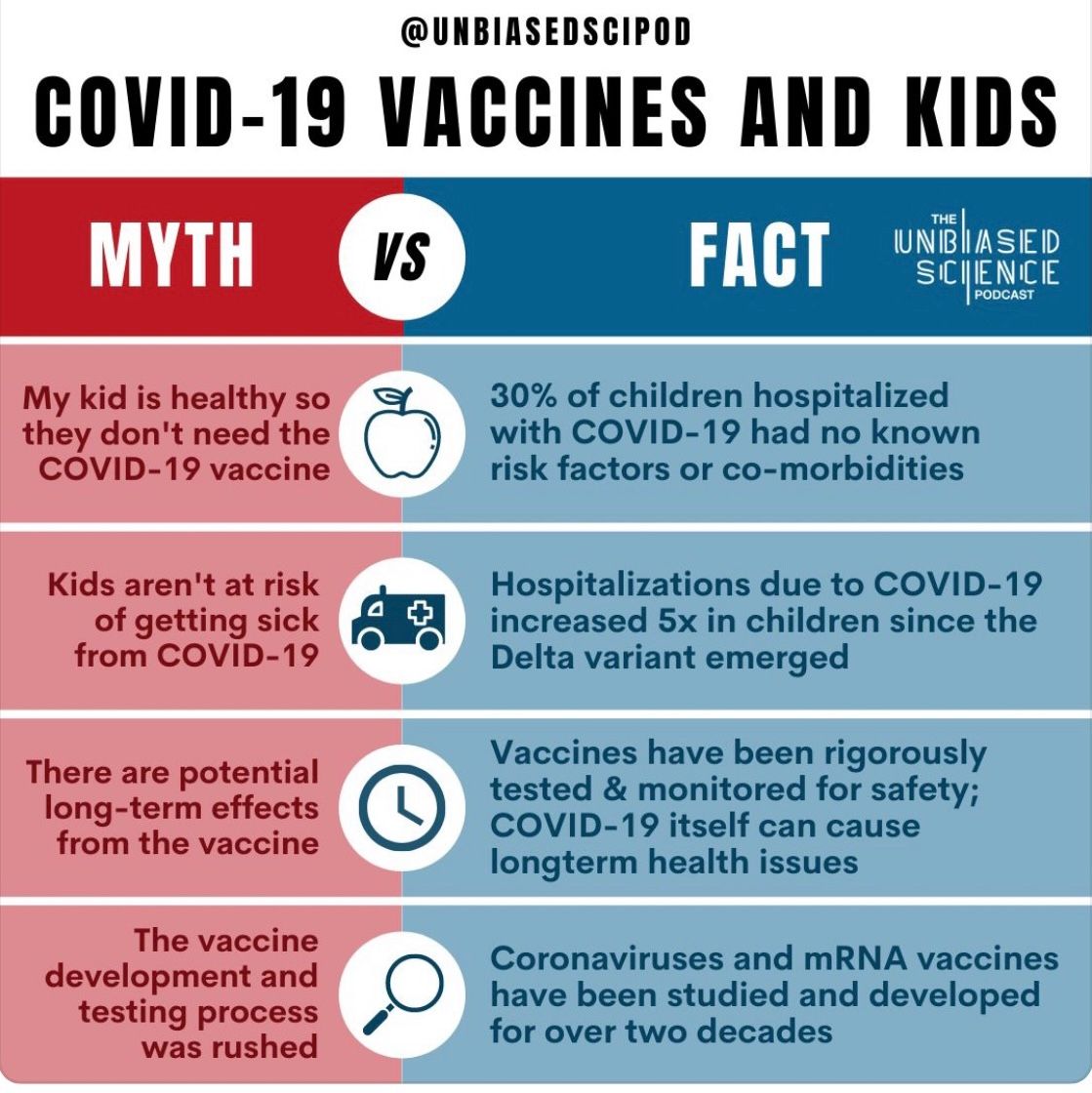
The final approval of the Pfizer-BioNTech pediatric vaccine for ages 5-11 years old has been granted by federal health experts with the FDA and CDC. Below, we answered common questions to ensure in-depth understanding of the vaccine for younger children. Vaccines hold a controversial conversation, making it important to stay updated with the COVID-19 vaccine development and concerns for parents choosing to protect their children.
What kind of dosage is it?
The pediatric dose is ⅓ (one third) of the normal adult dose. Children 5-11 will be vaccinated with two 10 microgram doses given 21 days apart.
What kind of symptoms will a child have?
Some children may not experience any symptoms at all. Symptoms in children can be similar to those in adults, such as slight fever, muscle pain, aches, nausea, and fatigue a couple days after injection.
Have vaccine trials on children of this age proven its safety and efficacy?
Yes, clinical trials have been performed on more than 3,000 pediatric participants within this age group. The vaccine was shown to be 90.7% effective against symptomatic COVID-19. In addition, the trial saw a clinical insignificance of serious reactions to the vaccine, such as anaphylactic attacks or heart inflammation/failure. Most cases of myocarditis (heart inflammation) have been detected outside this age group, primarily in males above 20 years old.
Heart failure and myocarditis were also observed to be much more common from contracting a natural infection and having COVID-19 than from receiving the vaccine.
How exactly did the FDA determine the effectiveness of this vaccine on young children?
The FDA makes decisions through analyzing evidence and weighing risks versus benefits. Clinical studies including thousands of pediatric participants receiving a small dose of the vaccine have been observed. In one study, around 1,305 children were given the vaccine and 663 were given a placebo. In the group of children who had taken the vaccine, only 3 cases of COVID-19 were reported. After the second dose, there were 0 cases. In contrast, those who had taken the placebo saw 16 cases of COVID-19 both before and after the second dose, totalling to 32 cases out of 663 pediatric patients. Health experts saw this comparison of 4.8% in respect to the 0.2% who received the vaccine, and concluded that benefits outweighed the risks.
How many children have been affected with COVID-19 overall? Is it even a big enough issue to consider giving children the vaccine?
Yes, COVID-19 has a huge impact on children and the community around them. About 1.9 million pediatric patients from 5-11 years old have been affected in the United States. About 8,000 of them have been hospitalized and 93 have died. While these numbers may not seem large, vaccinating young children is an essential first step in building herd immunity. Young children are often very careless regarding hygiene and are the most likely to transmit bodily fluids containing the COVID-19 virus to others, even without life threatening symptoms.
With the fast-paced landscape of COVID-19 vaccine testing, times can be stressful for patients choosing to immunize their child. However, it has been proven to be the easiest and efficacious way to protect your child, family, and those around you. The science, technology, and most updated data strongly shows promising benefits exceedingly outweighing risks of severe side effects. Vaccines, especially the COVID-19 vaccines, are easy, free, and more efficient in obtaining immunity.